
AMPARO®: Antimicrobial Motif Platform for Ameliorate Immune Response Outcomes
AMPARO® is an innovative approach that will interrupt the binding of viral particles to the target tissue or cell by interference before or after the viral adhesion process. Biocompatible nanoparticles functionalized with a recombinant human mannose-binding lectin (rhMBL) assume a nanostructured platform (AMPARO) capable of binding to various microorganisms with greater avidity, avoiding over-stimulation of the immune response.
Why did we choose to develop this solution?
Immune memory after the first contact with a virus or other microorganism can vary in relation to the immunity time and can also fail to protect the host. Highly specific characteristics of vaccines and some antiviral drugs limit these approaches in some contexts. The most important pandemics are caused by viruses with highly polymorphic genomes or microorganisms with very efficient routes of transmission.
The specific sequence of recombinant human MBL in this formulation is unable to activate the complement system while preserving its ability to bind viruses and bacterial organisms of medical importance. The platform creates a new concept to gain the function of the molecule, modifying its structure using a biocompatible polymeric component.

Scientific Basis
There are two types of immune response, an innate, more primitive and quick enough to deal with all the solutions in the body from scarring to tissue damage and infections. The second is an adaptive immunity, evolutionarily recent, that mainly regulates the intensity of the response and creates an adjusted and protective response in the secondary contacts. In cases where these adjustments (innate response) fail, harmful immunological results, such as allergies or diseases mediated by immunopathology, can be experienced.
An intricate network of cytokines and growth factors produced by immune and non-immune cells coordinates tissue repair after events of aggression, even in a non-infectious context. The mannan-binding lectin (MBL) is a standard molecule of innate immunity that participates in the repair mechanisms modulating the expression of cytokines and eliminating damaged cells, as well as microorganisms, controlling inflammatory signals. MBL’s role in protecting against fungal and bacterial infections is well demonstrated.
Regarding viral infections, MBL appears to have a controversial role, which has limited its use. This is due to its pluripotent characteristic of being a carbohydrate-binding molecule capable of inducing phagocytosis (opsonization), the movement of molecules into the cell and the activation of the complement system. Therefore, the balance of MBL activity, providing the enhancement of its antiviral immune resource, in consortium with the prevention of complement activation, provides the concept of the AMPARO nanoarray.
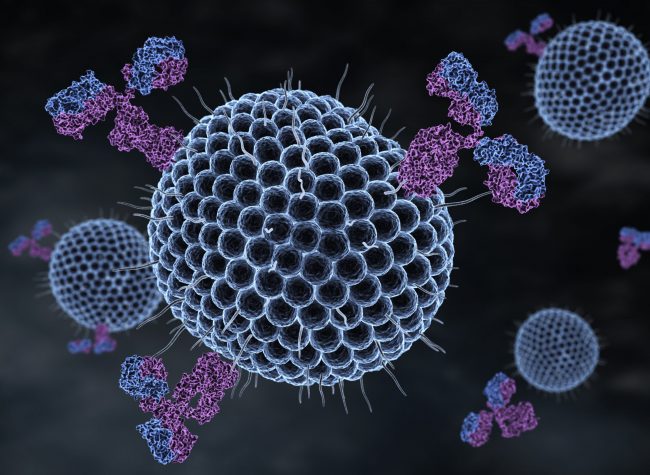
The antiviral activity of MBL is linked to the carbohydrate-binding domain [Kase et al. 1999, Leikina et al., 2005, Kawai et al., 2007, Avirutnan et al., 2010]. MBL binds the SARS-S pseudotype and blocks the interaction of the glycoprotein S with DC-SIGN and interferes with the initial stage of post-receptor interaction during viral entry [Zhou et al., 2010]. Clinical studies on COVID19 reveal the association between activation of the complement system and the pathophysiology of the disease [Jodele; Köhl 2020]. Therefore, our solution is focused on exploring the binding activity of recombinant human MBL as an antiviral property, while the molecule was designed to prevent complement activation. This approach is based on poly (lactic-co-glycolic acid) nanoparticles (PLGA) loaded with recombinant human MBL [Quadros et al., 2020]. Since then, virions have been eliminated by the PLGA MBL-nanoarray, reducing infectivity and the number of virus particles. It is important to note that PLGA is a polymer approved by the FDA for human use due to its biocompatibility. In addition, PLGA is biodegradable and exhibits adjustable mechanical properties.

